In the Lewis model for the HCl water reaction explain why you draw the arrow pointing from O to H. Notice that velocity always points in the direction of motion in other words for a curved path it is the tangent vectorLoosely speaking first order derivatives are related to.
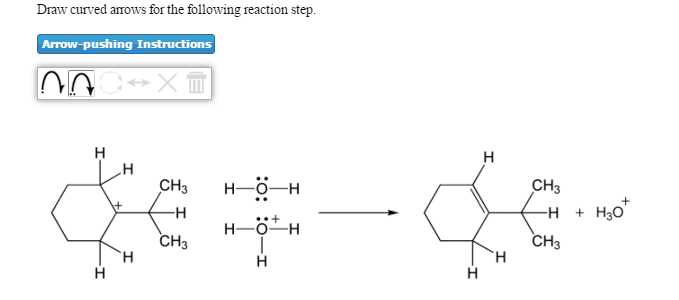
Solved Draw Curved Arrows For The Following Reaction Step Chegg Com
Image Draw the mechanism including curved arrows for the.

. A bond forms a bond breaks and thats the end of the reaction. The reaction of malic acid with PCl 5 leading to inversion of stereochemistry is an example of what we now call the S N 2 reaction and Walden was the first to make the observation that the stereochemistry is invertedIn fact the process of stereochemical inversion observed during the S N 2 reaction is sometimes called Walden. Provide a mechanism for the following.
The problem with this from a measurement standpoint is that we often cant determine an equilibrium constant for a reaction. How can we understand this. Lets break down all the steps in the following S N 1 reaction by looking at the energy diagram.
Following a few steps to draw Lewis structures Formal Charges and a table to quickly identify them Resonance Structures Resonance hybrid Curved Arrows and the rules for drawing resonance Structures a Complete Guide for Assessing the Relative Importance of Resonance Structures considering all the factors for stability of cations and anions. In this rate-determining step a. Many reactions of nucleophiles are not reversible.
Step 1 Breaking the C LG bond. From the instantaneous position r rt instantaneous meaning at an instant value of time t the instantaneous velocity v vt and acceleration a at have the general coordinate-independent definitions. And if we cant do that then we cant develop a reactivity scale based on equilibria.
Using the reaction of HCl and water as an example we use the curved arrow notation to denote how the electrons move between base and acid. The next logical step in expanding our ideas about Lewis acids and bases is to consider. The fast reaction of the carbocation with the nucleophile is the driving force of the S N 1 reaction since it pulls the equilibrium to the right according to the Le Châteliers principle.
S N 1 A Two-Step Mechanism. Note that the double arrows indicate that this step is reversible and moves quite fast.
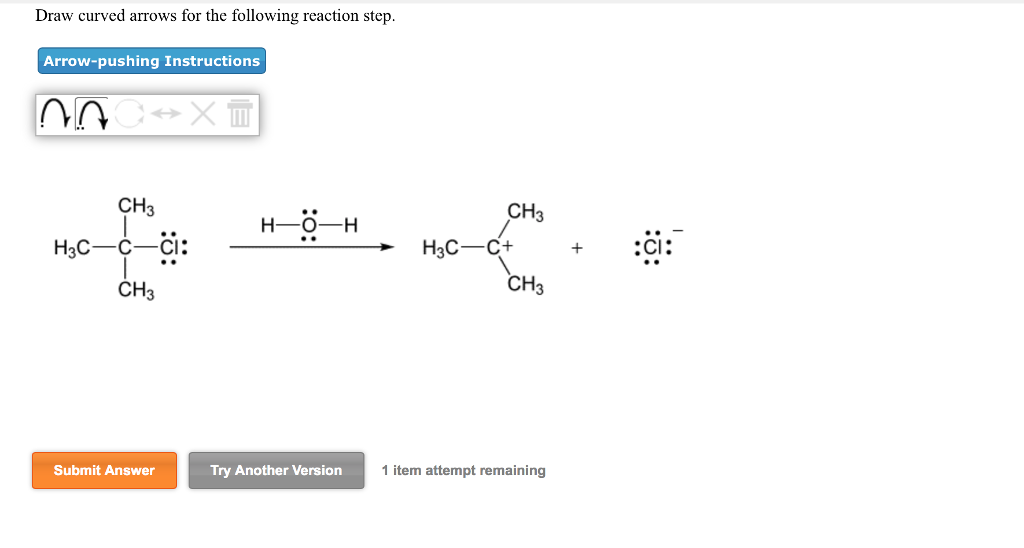
Solved Draw Curved Arrows For The Following Reaction Step Chegg Com
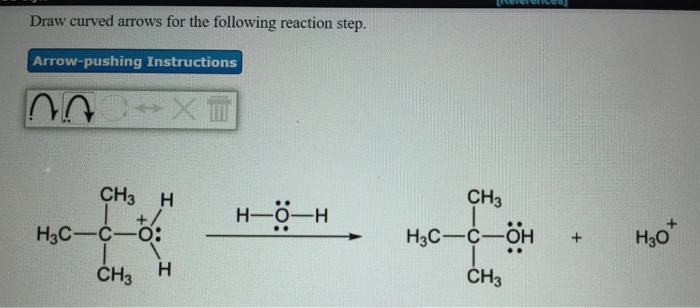
Solved Draw Curved Arrows For The Following Reaction Step Chegg Com
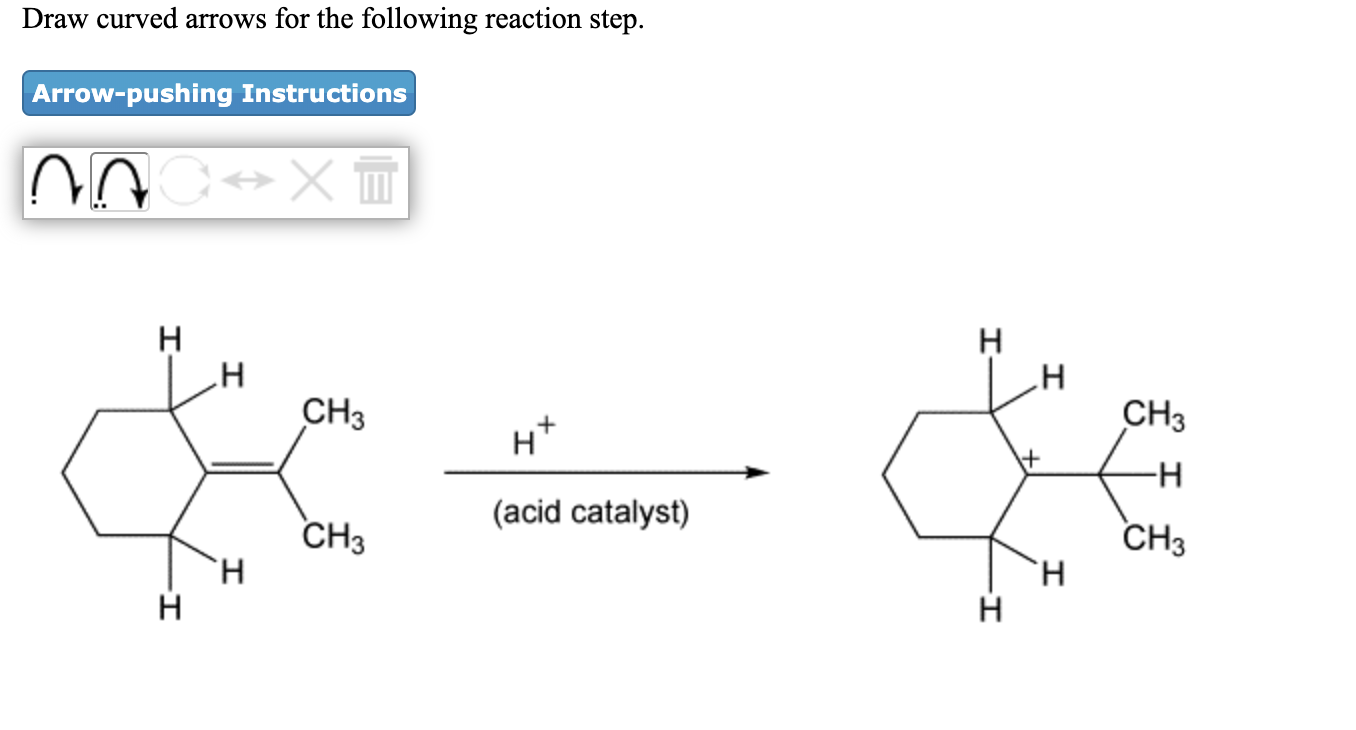
Solved Draw Curved Arrows For The Following Reaction Step Chegg Com
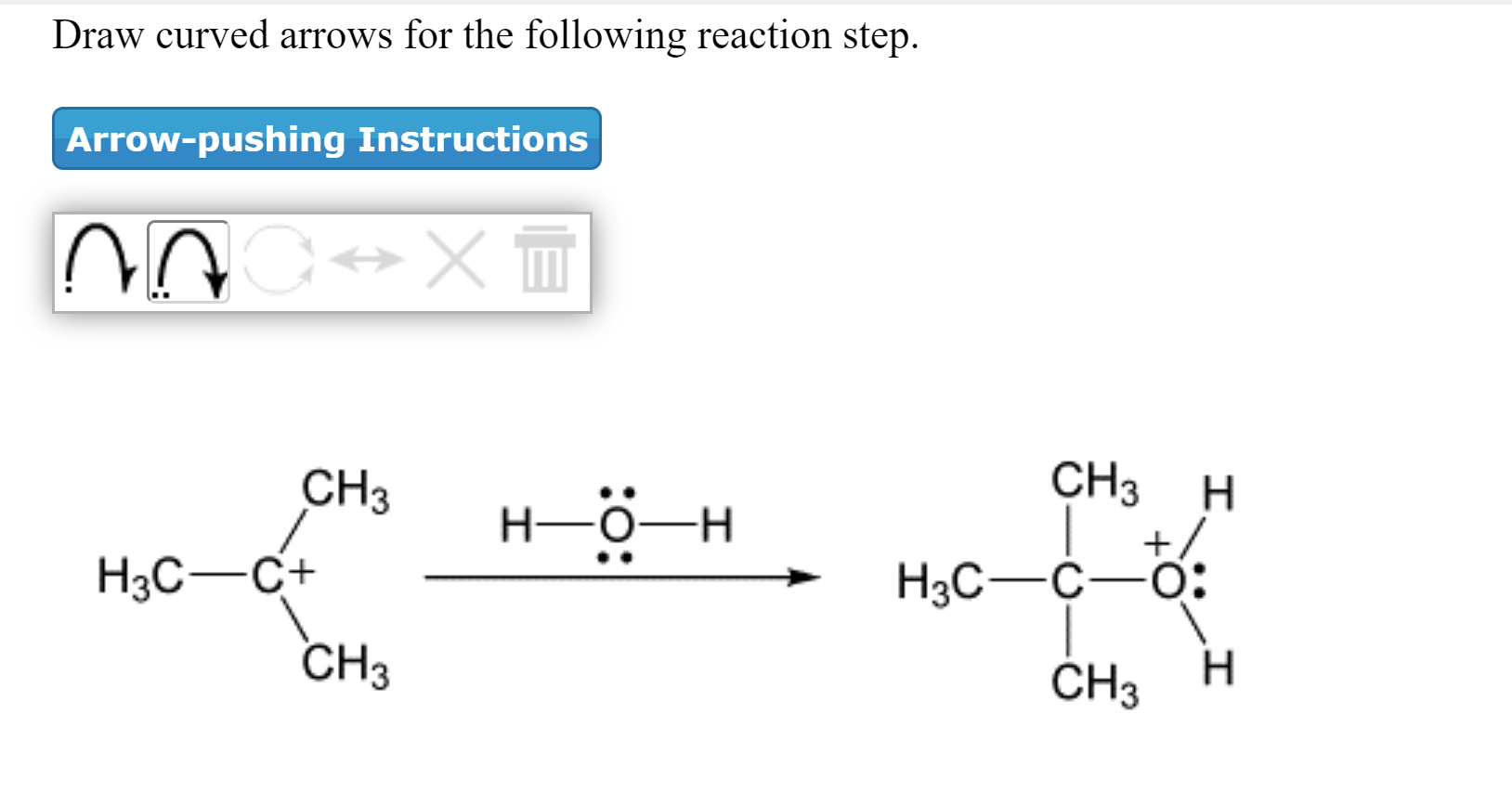
Solved Draw Curved Arrows For The Following Reaction Step Chegg Com
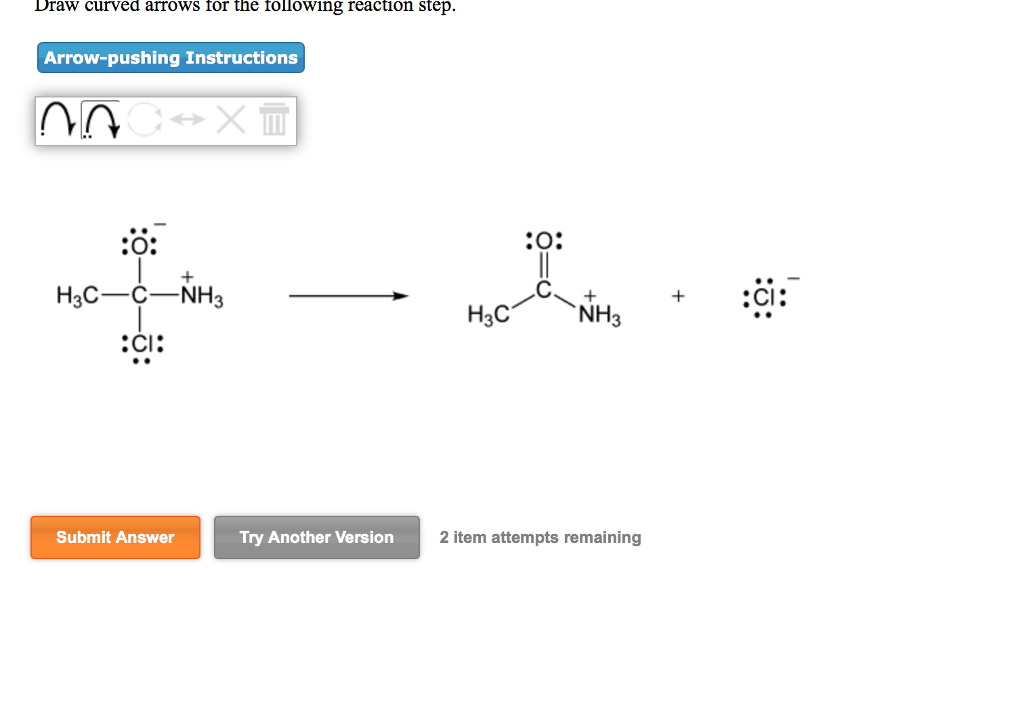
Solved Draw Curved Arrows For The Following Reaction Step Chegg Com
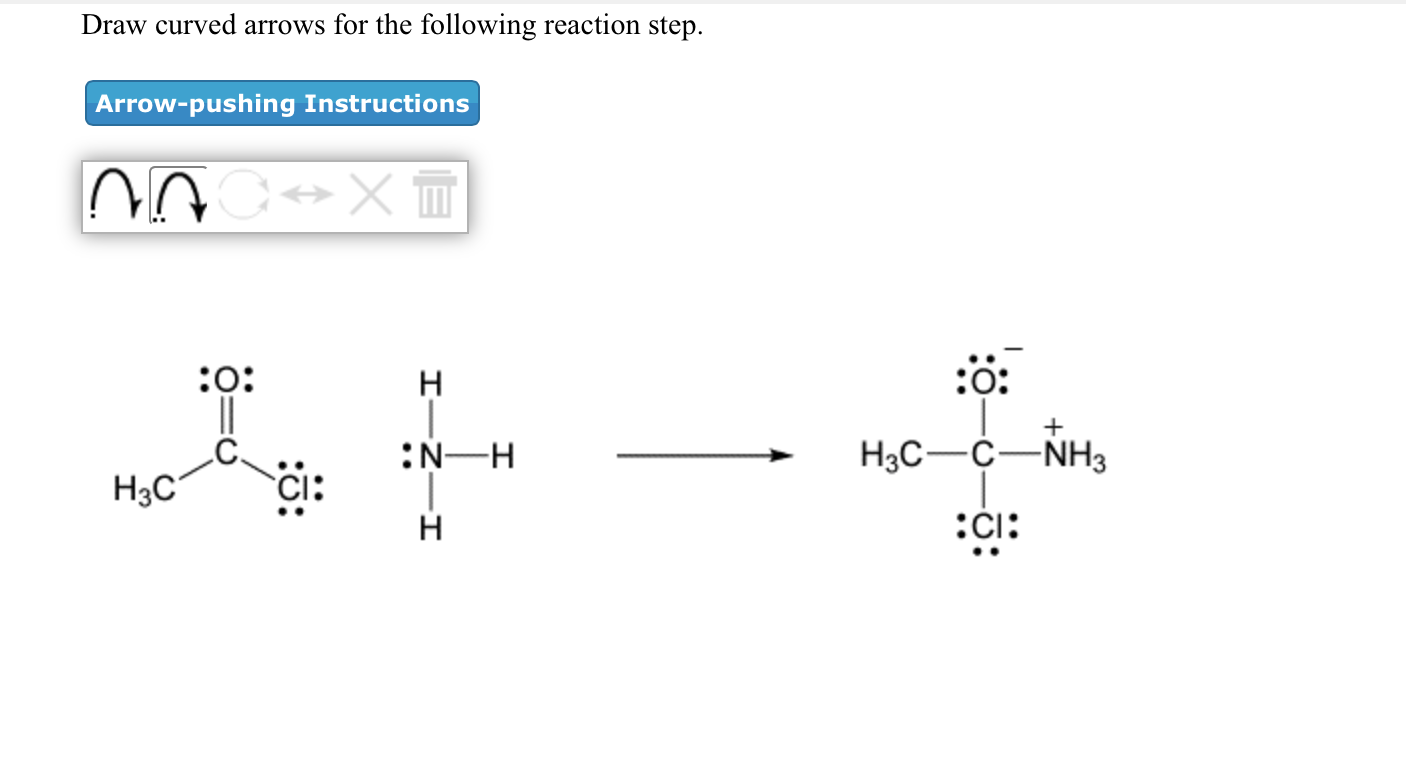
Solved Draw Curved Arrows For The Following Reaction Step Chegg Com

Solved Draw Curved Arrows For The Following Reaction Step Chegg Com

Solved Draw Curved Arrows For The Following Reaction Step Chegg Com
0 comments
Post a Comment